Introduction
Temperature is one of the most basic variables used to describe the state of the atmosphere. Air temperature varies with time from one season to the next, between day and night, and even from one hour to the next. Air temperature also varies from one location to another, from high altitudes and latitudes to low altitudes and latitudes. Temperature can be critical to some flight operations. As a foundation for the study of temperature effects on aviation and weather, this chapter describes temperature, temperature measurement, and heat transfer and imbalances.
Matter
Matter is the substance of which all physical objects are composed. Matter is composed of atoms and molecules, both of which occupy space and have mass. The Earth’s gravity acting on the mass of matter produces weight.
Energy
Energy is the ability to do work. It can exist in many forms and can be converted from one form to another. For example, if a ball is located at the edge of a slide, it contains some amount of potential energy (energy of position). This potential energy is converted to kinetic energy (energy of motion) when the ball rolls down the slide. Atoms and molecules produce kinetic energy because they are in constant motion. Higher speeds of motion indicate higher levels of kinetic energy.
Heat
Heat is the total kinetic energy of the atoms and molecules composing a substance. The atoms and molecules in a substance do not all move at the same velocity. Thus, there is actually a range of kinetic energy among the atoms and molecules.
Temperature
Temperature is a numerical value representing the average kinetic energy of the atoms and molecules within matter. Temperature depends directly on the energy of molecular motion. Higher (warmer) temperatures indicate a higher average kinetic energy of molecular motion due to faster molecular speeds. Lower (colder) temperatures indicate a lower average kinetic energy of molecular motion due to slower molecular speeds. Temperature is an indicator of the internal energy of air.
Temperature Measurement
A thermometer is an instrument used to measure temperature. Higher temperatures correspond to higher molecular energies, while lower temperatures correspond to lower molecular energies.
Temperature Scales
Many scientists use the Kelvin (K) scale, which is a thermodynamic (absolute) temperature scale, where absolute zero, the theoretical absence of all thermal energy, is 0 K. Thus, the Kelvin scale is a direct measure of the average kinetic molecular activity. Because nothing can be colder than absolute zero, the Kelvin scale contains no negative numbers.
The Celsius (°C) scale is the most commonly used temperature scale worldwide and in meteorology. The scale is approximately based on the freezing point (0 °C) and boiling point (100 °C) of water under a pressure of one standard atmosphere (approximately sea level). Each degree on the Celsius scale is exactly the same size as a degree on the Kelvin scale.
In the early 1990s, the United States aligned with ICAO standards by moving to the metric system for aviation weather reports. While some websites and applications provide temperature from the METAR in degrees Fahrenheit, these are done by the conversion software, as the temperature in the METAR is strictly reported in degrees Celsius. The United States uses the Fahrenheit (°F) scale for everyday temperature measurements for non-aviation purposes. In this scale, the freezing point of water is 32 °F and the boiling point is 212 °F.
See Table for conversion information between temperature scales.
Table . Celsius Temperature Conversion Formulae
| From Celsius | To Celsius | |
| Fahrenheit | [°F] = ([°C] × 9/5) + 32 | [°C] = ([°F] – 32) × 5/9 |
| Kelvin | [K] = [°C] + 273.15 | [°C] = [K] – 273.15 |
| For temperature intervals rather than specific temperatures: ±1 °C = ±1 K = ±1.8 °F | ||
Table . Fahrenheit Temperature Conversion Formulae
| From Fahrenheit | To Fahrenheit | |
| Celsius | [°C] = ([°F] – 32) × 5/9 | [°F] = ([°C] × 9/5) + 32 |
| Kelvin | [K] = ([°F] + 459.67) × 5/9 | [°F] = ([K] × 9/5) – 459.67 |
| For temperature intervals rather than specific temperatures: ±1 °F = ±.56 °C = ±.56 K | ||
A thermometer changes readings due to the addition or subtraction of heat. Heat and temperature are not the same, but they are related.
Figure . gives a comparison of Kelvin, Celsius, and Fahrenheit temperature scales.
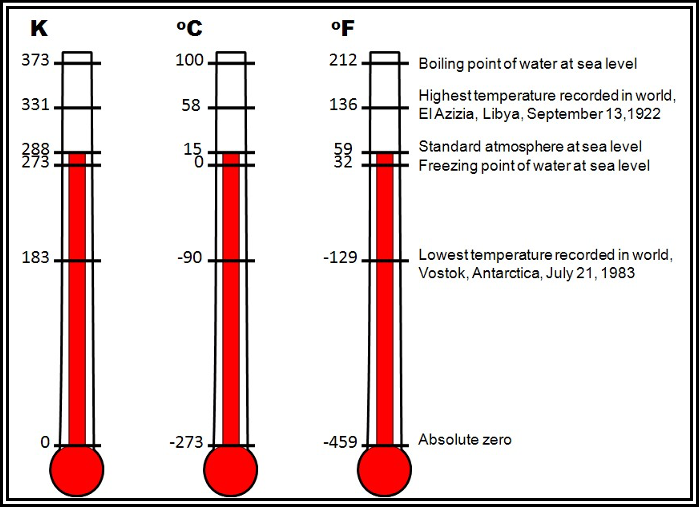
Figure . Comparison of Kelvin, Celsius, and Fahrenheit Temperature Scales
Heat Transfer
Heat transfer is energy transfer as a consequence of temperature difference. When a physical body (e.g., an object or fluid) is at a different temperature than its surroundings or another body, transfer of thermal energy, also known as heat transfer (or heat exchange), occurs in such a way that the body and the surroundings reach thermal equilibrium (balance). Heat transfer always occurs from a hot body to a cold body. Where there is a temperature difference between objects in proximity, heat transfer between them can never be stopped; it can only be slowed down.
The heat source for the surface of Earth is the Sun. Energy from the Sun is transferred through space and through the Earth’s atmosphere to the Earth’s surface. Since this energy warms the surface and atmosphere, some of it becomes heat energy. There are three ways heat is transferred into and through the atmosphere: radiation, conduction, convection, or any combination of these. Heat transfer associated with the heat change of water from one phase to another (i.e., liquid water absorbs heat when changed to a vapor and liquid water releases heat when it changes to ice) can be fundamentally treated as a variation of convective heat transfer. The heat transfer associated with water will be discussed in Chapter 6, Water Vapor.
Radiation
If a person has ever stood in front of a fireplace or near a campfire, then they have felt the heat transfer known as radiation. The side of the body nearest the fire warms, while the other side remains unaffected by the heat. Although people are surrounded by air, the air has nothing to do with this type of heat transfer. Heat lamps that keep food warm work in the same way.
Radiation is the transfer of heat energy through space by electromagnetic radiation. These electromagnetic waves travel at the speed of light and are usually described in terms of wavelength or frequency. Frequencies range from gamma rays on the high end to radio waves on the low end. Also contained in the spectrum are x ray, ultraviolet, visible, infrared, and microwave.

Figure . Radiation Example
All objects emit (radiate) energy as the heat energy within the object is converted to radiation energy. This transmitted radiation passes through entities such as air, water, or space. Along the way, the radiation can be reflected, which occurs when the wave energy changes direction when encountering an object. Eventually, the radiation is absorbed and the electromagnetic wave energy is converted to heat energy by the absorbing object. The emitting object loses heat energy, and the absorbing object gains heat energy during this process.
Solar and Terrestrial Radiation
All objects emit radiation energy, including the Sun (solar radiation) and the Earth (terrestrial radiation). An object’s wavelength of maximum radiation is inversely related to its temperature; the hotter (colder) the object, the shorter (longer) the wavelength. The Sun’s wavelength of maximum radiation is relatively short and is centered in the visible spectrum. The Earth’s wavelength of maximum radiation is relatively long and is centered in the infrared spectrum.
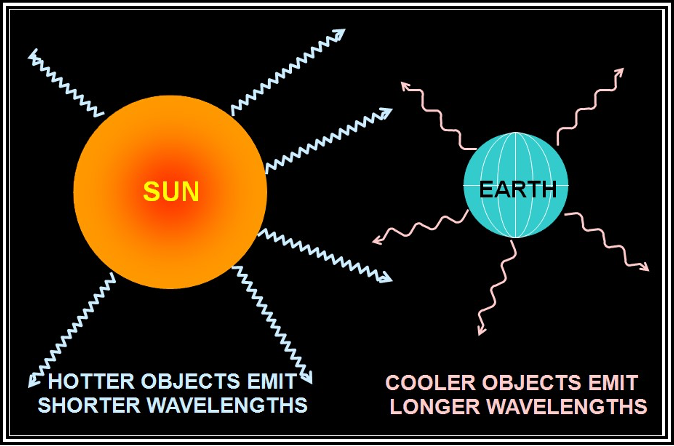
Figure . Temperature’s Effect on Radiation Wavelength
Some of the solar radiation that reaches the Earth’s surface is radiated back into the atmosphere to become heat energy. Dark-colored objects such as asphalt absorb more of the radiant energy and warm faster than light-colored objects. Dark objects also radiate their energy faster than light-colored objects.
Solar Zenith Angle
The intensity of incoming solar radiation that strikes the Earth’s surface (insolation) varies with solar zenith angle. Solar zenith angle is the angle measured from the Earth’s surface between the Sun and the zenith (i.e., directly overhead). Solar zenith angle varies with latitude, season, and the diurnal cycle (sunrise/sunset). Figure 5-4 illustrates this concept. Insolation is maximized when the solar zenith angle is zero degrees (0°), which means the Sun is directly overhead. With increasing solar zenith angle, the insolation is spread over an increasingly larger surface area (y is greater than x) so that the insolation becomes less intense. Also, with increasing solar zenith angle, the Sun’s rays must pass through more of the Earth’s atmosphere, where they can be scattered and absorbed before reaching the Earth’s surface. Thus, the Sun can heat the surface to a much higher temperature when it is high in the sky, rather than low on the horizon.
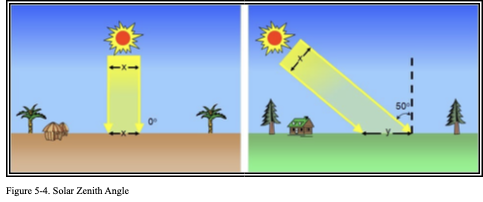
Figure. Solar Zenith Angle
Conduction
Conduction is the transfer of energy (including heat) by molecular activity from one substance to another in contact with or through a substance. Heat always flows from the warmer substance to the colder substance. The rate of heat transfer is greater with larger temperature differences and depends directly on the ability of the substance(s) to conduct heat. During conduction, the warmer substance cools and loses heat energy, while the cooler substance warms and gains heat energy. Heat (thermal) conductivity is the property of a substance that indicates its ability to conduct heat as a consequence of molecular motion. Units are watts per meter-kelvin (W m-1 K-1). Table 5-3 provides the heat (thermal) conductivity of various substances. Note that air is a poor thermal conductor.
Table . Heat (Thermal) Conductivity of Various Substances
| Material | Phase | Heat (Thermal) Conductivity (W m-1 K-1) |
| Silver | Solid | 429 |
| Copper | Solid | 401 |
| Aluminum | Solid | 250 |
| Iron | Solid | 80 |
| Sand (saturated) | Solid | 2.7 |
| Water (ice) | Solid (0 °C) | 2.18 |
| Sandstone | Solid | 1.7 |
| Limestone | Solid | 1.26–1.33 |
| Glass | Solid | 1.05 |
| Water (liquid) | Liquid | 0.58 |
| Sand (dry) | Solid | 0.35 |
| Soil | Solid | 0.17–1.13 |
| Wood (oak) | Solid | 0.17 |
| Wood (balsa) | Solid | 0.055 |
| Snow | Solid (<0 °C) | 0.05–0.25 |
| Air | Gas | 0.024 |
| Water (steam) | Gas (125 °C) | 0.016 |
| All measurements are at 25 °C unless otherwise noted. Note: 1 K equals -272.15 °C. | ||
Convection
Convection is the transport of heat within a fluid, such as air or water, via motions of the fluid itself. This type of heat flow takes place in liquids and gases because they can move freely and it is possible to set up currents within them. Water boiling in a pot is an example of convection. Because air is a poor thermal conductor, convection plays a vital role in the Earth’s atmospheric heat transfer process. Figure illustrates examples of various heat transfer processes.
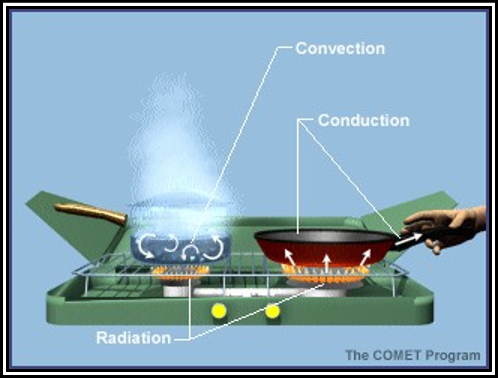
Figure . Heat Transfer Examples
Thermal Response
Whether by radiation, conduction, convection, or a combination of these, the temperature response to the input (or output) of some specified quantity of heat varies from one substance to another. Specific heat capacity, also known simply as specific heat, is defined as the measure of heat energy required to increase the temperature of a unit quantity of a substance by a certain temperature interval. Specific heat capacity is typically expressed in units of joules per gram-kelvin (J g-1 K-1). Thus, two different substances with identical temperature measurements do not necessarily possess the same amount of heat energy. When exposed to the same amount of heat energy, a substance with a low specific heat capacity warms up more than a substance with a higher specific heat capacity. Table lists the specific heat capacity of various substances.
Table . Specific Heat Capacity of Various Substances
| Substance | Phase | Specific Heat Capacity (J g-1 K-1) |
| Water (steam) | Gas (100 °C) | 4.22 |
| Water | Liquid (25 °C) | 4.18 |
| Wood (balsa) | Solid | 2.90 |
| Water (ice) | Solid (0 °C) | 2.05 |
| Wood (oak) | Solid | 2.00 |
| Soil (wet) | Solid | 1.48 |
| Sandy clay | Solid | 1.38 |
| Air (sea level, dry) | Gas | 1.01 |
| Asphalt | Solid | 0.92 |
| Clay | Solid | 0.92 |
| Aluminum | Solid | 0.91 |
| Brick (common) | Solid | 0.90 |
| Concrete | Solid | 0.88 |
| Glass | Solid | 0.84 |
| Limestone | Solid | 0.84 |
| Sand (quartz) | Solid | 0.83 |
| Soil (dry) | Solid | 0.80 |
| Granite | Solid | 0.79 |
| Iron | Solid | 0.46 |
| Copper | Solid | 0.39 |
| Mercury | Liquid | 0.14 |
| Lead | Solid | 0.13 |
| All measurements are at 25 °C unless otherwise noted. Note: 1 K equals -272.15 °C. | ||
Water has the highest specific heat capacity of any naturally occurring substance. That means it has a much higher capacity for storing heat energy than other substances, such as soil, sand, rock, or air. Water can store large amounts of heat energy while only experiencing a small temperature change. Figure compares the specific heat capacity of water and sand. The specific heat capacity of water is more than five times that of quartz sand. Thus, 4.18 J of heat are required to raise the temperature of 1 gram (g) of water by 1 °C, while only 0.83 J are required to raise the temperature of 1 g of quartz sand by 1 °C. This is one reason why beach sand is hotter than water on a sunny, summer afternoon.
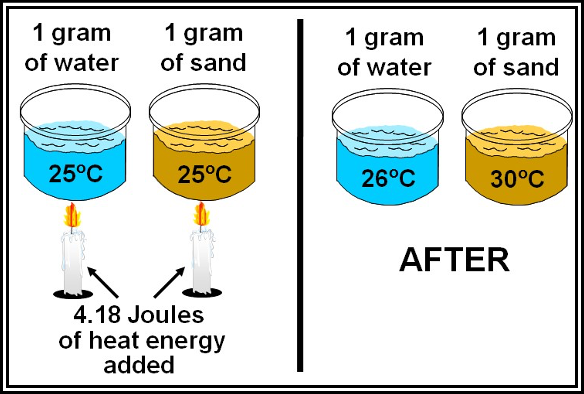
Figure . Specific Heat Capacity: Water versus Sand
The difference in specific heat capacities is one of the primary reasons why the temperature of a body of water, such as a lake or the ocean, is less variable with time than the surface temperature of land. Water heats up more slowly than land during the day and during the summer, and cools down more slowly at night and during the winter. Thus, a body of water exhibits greater resistance to temperature change (called thermal inertia) than does a land mass. Heat flow differences are another reason why water bodies warm up and cool down more slowly than land. Incoming solar radiation penetrates water to significant depths, but can only heat the top skin layer of soil and rock. Also, since water is a fluid, its heat energy can be circulated through great volumes and depths via convection. Water temperature changes occur to depths of 6 meters (m) (20 ft) or more on a daily basis, and 200 to 600 m (650 to 1950 ft) annually. The process is more problematic over land since heat must be transferred via the slow process of conduction. Land temperature changes occur to depths of only 10 centimeters (cm) (4 inches (in)) on a daily basis and 15 m (50 ft) or less annually. Water is much more resistant to temperature changes than land. It warms up and cools down more slowly than land and helps to moderate nearby air temperature. This is why islands and localities located immediately downwind from the ocean or a large lake (maritime locations) exhibit smaller diurnal and seasonal temperature variations than localities well inland (continental locations). Figure 5-7 illustrates this effect. Although both cities are at approximately the same latitude, the temperature is much less variable in San Francisco (maritime) than St. Louis (continental).
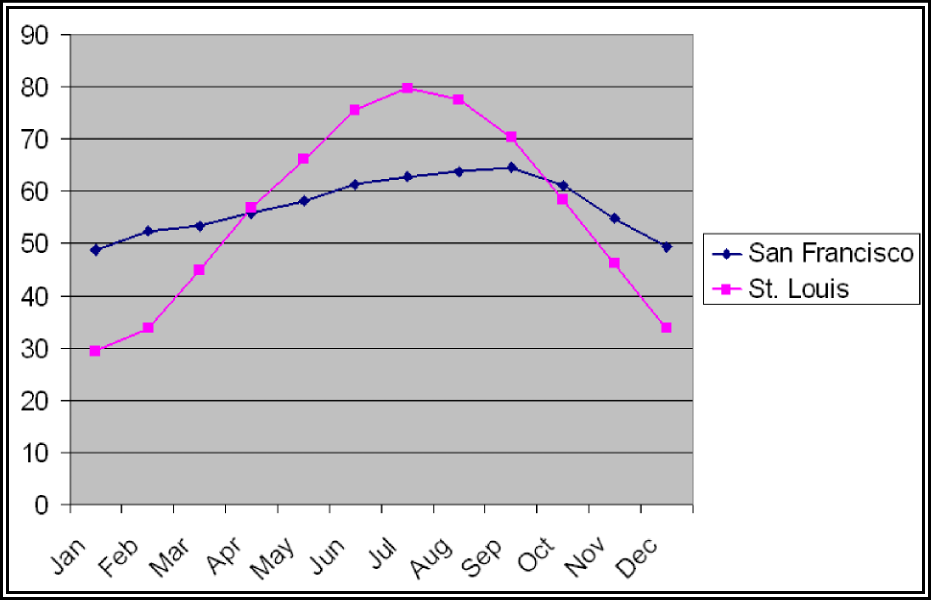
Figure . Variation of Mean Daily Temperatures for San Francisco (Maritime) and St. Louis (Continental)
Temperature Variations with Altitude
A lapse rate of temperature is defined as a decrease in temperature with height. In Figure 4-2, it was stated that the temperature decreases 6.5 °C/km (3.57 °F/1,000 ft) in the standard atmosphere. But since this is an average, the exact value seldom exists. In fact, temperature in the troposphere sometimes remains constant or even increases with height. Caution should be taken when using the standard lapse rate to estimate the freezing level. Quite often the boundary layer is dry adiabatic and the estimate of freezing level could be in error.
Atmospheric Sounding
An atmospheric sounding, or simply sounding, is a plot of the vertical profile of one or more atmospheric parameters, such as temperature, dewpoint, or wind above a fixed location. Soundings are used extensively by meteorologists to determine the state of the atmosphere.
Isothermal Layer
An isothermal layer is a layer within the atmosphere where the temperature remains constant with height.
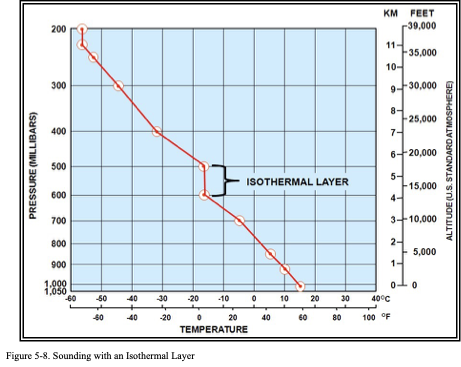
Figure . Sounding with an Isothermal Layer
Temperature Inversion
A temperature inversion, or simply inversion, is a layer in which the temperature increases with altitude. If the base of the inversion is at the surface, it is termed a surface-based inversion. If the base of the inversion is not at the surface, it is termed an inversion aloft (see Figure 5-9).
A surface-based inversion typically develops over land on clear nights when wind is light. The ground radiates and cools much faster than the overlying air. Air in contact with the ground becomes cool, while the temperature a few hundred feet above changes very little. Thus, temperature increases with height.
An inversion may also occur at any altitude when conditions are favorable. For example, a current of warm air aloft overrunning cold air near the surface produces an inversion aloft. Inversions are common in the stratosphere.
The principal characteristic of an inversion layer is its marked stability, so that very little turbulence can occur within it. Turbulence will be discussed at length in , Turbulence.
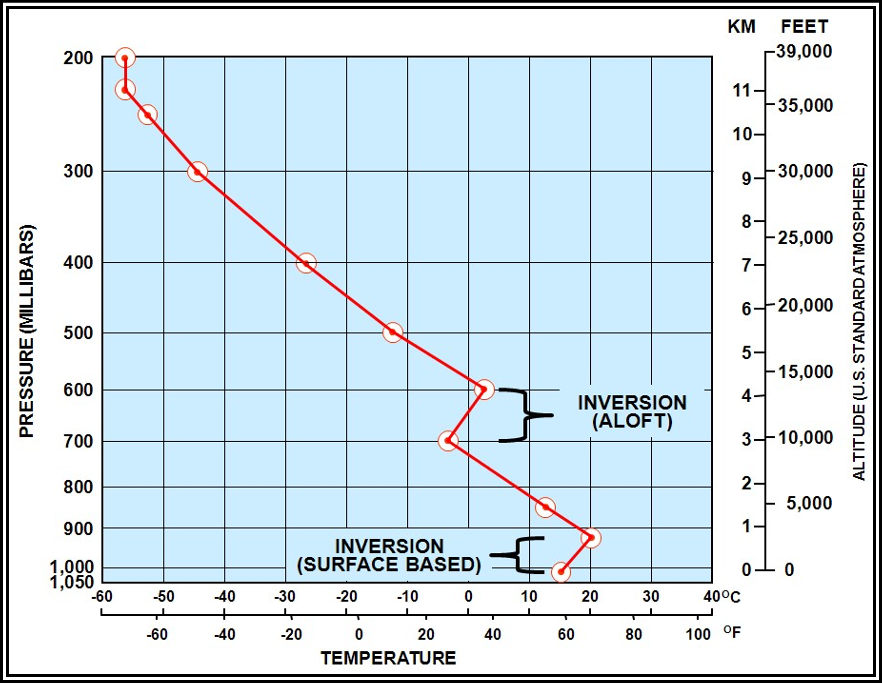
Figure . Sounding with a Temperature Inversion





